AstraZeneca has withdrawn its Covid-19 vaccine from the market
Pharmaceutical giant AstraZeneca has formally requested the European Medicines Agency to withdraw its approval of the COVID-19 vaccine.
In an update on the website of the European Medicines Agency on Wednesday, the regulator of the agency has explained that the authorization of the Uviko-19 vaccine Vaxzevria of AstraZeneca Company has been withdrawn at the request of the marketing authorization holder.
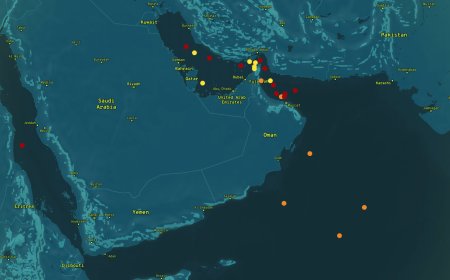
The Uviko-19 vaccine, which was originally approved in January 2021, faced safety concerns about rare blood clots, leading various countries to temporarily suspend its use.
The state of anxiety and doubting the Covid-19 vaccine of the Astrazeneca company persisted despite the European Union Regulator saying that the overall risk that can be caused by the vaccine is of a small level.
It will be remembered that when the world was affected by the Corona epidemic, billions of Astrazeneca vaccine doses were distributed in poor countries through a program coordinated by the United Nations because it was cheaper and easier to produce and distribute.

However, studies later suggested that RNA vaccines developed by Pfizer-BioNTech and Moderna offer better protection against Covid-19 and its many variants.